
Henry D. Young, Ph.D.
I'm a professor of Materials Science and Engineering at Wright State University. My research is primarily focused on developing novel materials processing methods and pushing the limits of existing materials properties. My work is often interdisciplinary and regularly crosses the lines of traditional engineering fields. I've made long-lasting contributions in these areas: growth of thin-film oxide heterostructures, development of novel electronic packaging materials, additive manufacturing of electronic materials, the study of fast laser-fluid interactions, rapid prototyping of biological and living materials, and laser powder bed fusion of metals. Recently, my research has focused on two separate areas: the intersection between additive manufacturing and traditional processes, and on novel transpirational surface protection strategies. As you can see, my interests are pretty broad.
Curriculum Vitae
CURRENT RESEARCH PROJECTS
Laser powder bed fusion of metal alloys
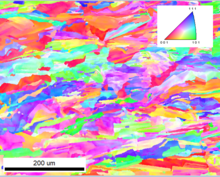
additively manufactured Inconel 718
Metal additive manufacturing (AM) is a new and rapidly growing field. It's become clear that metal AM methods are going to change the way that many components are manufactured, both at the cutting edge where otherwise impossible geometries and materials become accessible, and in the area of legacy technology where replacement parts can now be easily manufactured without the need for expensive tooling. However, there are still many questions regarding microstructure and mechanical properties that arise as a result of the unique thermal conditions that occur during AM. I study the microstructure of additively manufactured stainless steels, nickel-based superalloys and high-entropy alloys. From a scientific perspective, I'm particularly interested in the effects of high-speed deformation and impact on additively manufactured microstructures. From a pratical perspective, I think that there is an opportunity to use metal additive manufacturing to enhance traditional forging methods, especially in the area of preform design. AM preform design could significantly reduce forging die count, drasticallly speeding up the forging manufacturing process. Novel AM-facilited preform design could also lead to new forging strategies. Overall, I think that additive manufacturing is more likely to enhance and breathe new life into traditional metal manufacturing methods, rather than replace them entirely.
Nanosilver composite materials for electronic packaging applications
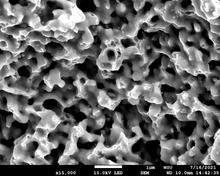
93% dense, fully ductile fracture
Silver is a really interesting metal. It has the highest electrical and thermal conductivity, and is very corrosion and oxidation resistant. Why isn't it used in more electronics and thermal management applications? Traditionally, it's avoided because of high cost, leading to extensive usage of copper. However, modern electronics technology has become so complex that the raw materials cost is often a tiny fraction of the total. It's time to re-evaluate silver for many electronics technologies. I'm especially interested in a) nanosilver-based electronics materials and b) silver as a cavity infill material for chiplet-based electronics packages. While the melting point of solid silver is 962 C, silver nanoparticles can melt at temperatures as low as 100 C (or even lower) and exhibit enhanced surface diffusion. This is fascinating from a scientific perspective. Practically, it means that silver nanopastes can be easily shaped and then consolidated to solid silver at temperatures achievable with a typical soldering iron. Thus, nanosilver could find use as a solder alternative, a high-performance semiconductor chip glue, and many other applications. I study the consolidation phenomena in these materials, their thermomechanical creep behavior at temperatures relevant to power electronics applications, and ways to formulate silver pastes with extremely low shringage during curing, in order to enable novel chip packaging strategies. In the future, I'd like to study the effect of alloying elements on nanosilver consolidation behavior, as a way of controlling the zero-stress point in electronics packages.
Laser Direct Writing of Multimaterial Electrical/Photonic Devices from Single Precursors

an active terahertz split ring resonator and a composite Cu/Ag logo,
The electronics industry needs new, versatile approaches to materials deposition and device manufacturing. I'm interested in developing laser-based methods to deposit electronic materials with submicron resolution. Our recent work in this area shows that controllable mixed oxide and metallic silver deposition can be achieved from solvated silver nitrate through adjustment of laser conditions: wavelength, intensity and pulse duration. This allows fabrication of devices with both metallic and oxide structures, with submicron resolution from a single precursor material. Traditionally, silver oxide formation is considered an unwanted phase. However, this represents a missed opportunity, since oxide deposition is an integral component of semiconductor device manufacturing. Our work suggests the prospect of depositing multiple materials from a single precursor: a novel approach that has received very little research focus. Recently, we have explored this research space, utilizing a mixed metal nitrate precursor system to achieve the direct-writing of composite Cu/Ag metal patterns, and extended this strategy to polymer/metal deposition using solvated metal salts in a polymer precursor. We also demonstrated functional terahertz-polarizer devices. Our work points toward a novel materials deposition strategy where a surprisingly wide palette of materials may be deposited from a single precursor. However, there are several fundamental scientific questions about deposition mechanisms that remain and require further study.
TEACHING AND TEACHING PHILOSOPHY
My teaching is primarily focused on training undergraduate engineers in both Materials and Mechanical fields. I regularly teach classes in Metallography, Mechanical Testing, Mechanical Properties of Materials, Introduction to Materials Engineering, Mechanical Metallurgy, Thermodynamics of Materials, Kinetics and Diffusion of Materials, and Powder Processing of Materials. I have also taught Solid Modeling and Solidworks, and have served as the organizer for the College of Engineering and Computer Science Ph.D. Seminar series. When I teach, I strive to present the proper ratio of theory and engineering practice. This ratio varies widely depending on the specific engineering topic. For example, Thermodynamics of Materials is probably 80% theory, while Powder Processing of Materials is the opposite. My lecture style involves a large amount of in-person board work, using both traditional whiteboard and tablet-projection approaches. Traditional computer presentation is used for graphical information, but is otherwise kept to a minimum (Powerpoint lectures put students to sleep). I query my classes frequently so that I can dynamically adjust my lecture content. I also use software-based engineering examples whenever appropriate. In practice, this means that my lectures often switch back and forth between computer and whiteboard. While this may be a staid approach, I've found that students vastly prefer this to other up-and-coming teaching strategies, and it seems to keep them effectively engaged. I welcome the time when online learning, MOOCs, flipped classrooms, gamified classes, etc. become more effective than the traditional university classroom. However, when it comes to teaching in-depth engineering, my impression is that these other methods aren't quite ready yet.
RECENT PUBLICATIONS
- J. M. Adams, D. M. Heligman, R. O’Dell, C. Y. Wang, D. Young, “Laser printing of silver and silver oxide,” Optics Express, 14 (2024) 2719. https://doi.org/10.1364/OME.540753
- E. Stang, C. Lesko, D. Young, “Constant Strain-Rate Garofalo Creep Behavior of Intermediate 83Pb/10Sb/5Sn/2Ag and 91.5Sn/8.5Sb Solder Materials,” J. Electronic Packaging, 146 (2024) 031008. https://doi.org/10.1115/1.3157651
- R. Tullis, A. Dunn, D. Young, N. Klingbeil, J. Gockel, “Additive Manufacturing Bulk Parameter’s Influence on Surface Roughness, Microstructure, and Fatigue” JOM, 75 (2023) 1975-1981, https://doi.org/10.1007/s11837-023-05779-6.
- Abdelal, N., Dib, N., Young, D., “Electromagnetic interference shielding and dielectric properties of graphene nanoplatelets/epoxy composites in the x-band frequency range,” J. Mater. Sci. (2022). https://doi.org/10.1007/s10853-022-07475-3
- J. R. McCoppin, M. S. Hanchak, L. J. Elston, D. Young, “Boil-Off Calorimetry Enthalpy Measurements and Equation of State of an Aqueous Pyridine Azeotrope,” Intl. Journal of Refrigeration (2021), doi:https://doi.org/10.1016/j.ijrefrig.2021.04.006
- J.R. McCoppin and D. Young, “Mass Transport, Creep and Zero-Stress-Point Shifting in a Nano-Silver Die Attach Material During Thermal Cycling,” Journal of Electronic Materials, 49 (2020) 3982-3989.
RESEARCH GROUP - 2023

