Mike Kemp, Ph.D.
Curriculum Vitae
Education History
EDUCATION
1999 B.S. in Biological Sciences, Wright State University, Dayton, Ohio
2006 Ph.D. in Biomedical Sciences, Wright State University, Dayton, Ohio
- Advisor: Michael Leffak, Ph.D.
POST-DOCTORAL TRAINING
2006-2011 DNA Repair and DNA Damage Signaling, University of North Carolina Department of Biochemistry & Biophysics, Chapel Hill, NC
- Advisor: Nobel Laureate Aziz Sancar, M.D., Ph.D.
2016-2017 Skin Biology and Aging, Wright State University Department of Pharmacology & Toxicology, Dayton, Ohio
- Advisor: Jeffrey Travers, M.D., Ph.D.
Research Statement
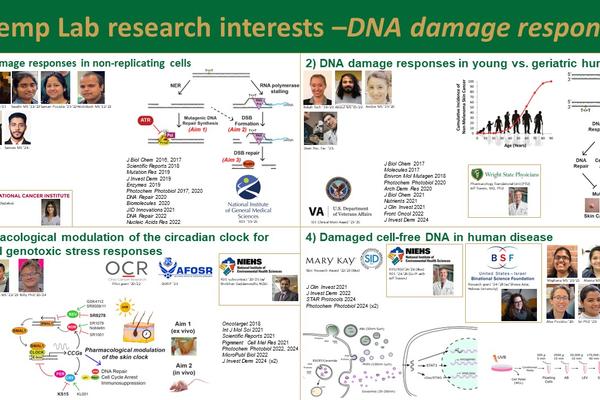
Overview of Research in the Kemp Laboratory: Research in my laboratory is in the general area of DNA damage and genome instability (also known as genetic toxicology). We seek to better understand how human cells respond to DNA damage caused by environmental carcinogens and anti-cancer agents so that we can develop new therapeutic approaches for preventing and treating cancers. We aim to achieve this goal through the following research projects:
Project 1: DNA damage responses in non-replicating human cells and tissues. Most of our understanding of how cells respond to DNA damage is based on model systems in which DNA replication and the cell cycle play prominent roles. However, given that most cells in the body are in a quiescent, terminally differentiated, or senescent state, we believe that new insights into mutagenesis and DNA damage responses will be gained by using non-replicating cell-based systems. We are particularly interested in a protein kinase known as ATR, which regulates DNA damage responses in replicating and cycling cells and has become an attractive target for anti-cancer therapies. This project was previously funded by an R01 grant from NIGMS.
Project 2: Circadian clock disruption and pharmacological modulation in response to environmental stressors. The body’s natural circadian rhythms impact many aspects of cellular biochemistry and physiology, including the response to DNA damage. The goal of this project is to determine whether recently discovered compounds that target protein components of the circadian clock transcriptional machinery can be used to modulate how normal and cancer cells respond to DNA damage caused by environmental carcinogens and anti-cancer drugs. We are also collaborating with researchers at WPAFB’s AFRL to extend this hypothesis to Air Force-relevant stressors. Finally, altered circadian rhythms due to rotating shift work may alter responses to UV radiation and other carcinogens and predispose individuals to cancer development, and thus we are working to compare DNA damage responses in the skin of day shift vs rotating shift individuals. This work was previously funded by a pilot project grant from Ohio Cancer Research Associates and by a sub-contract on a collaborator’s NIEHS R01. Funds from the Air Force Office of Scientific Research has also been obtained to purchase special equipment for monitoring circadian rhythms.
Project 3: Comparing DNA damage responses in young versus geriatric human skin. Most skin cancers are associated with exposure to UV wavelengths of sunlight and are known to primarily occur in individuals over the age of 60. Little is known regarding how the UVB DNA damage response may differ between young adult and geriatric skin. The purpose of this project is to examine the hypothesis that reduced levels of insulin-like growth factor-1 (IGF-1) due to increased fibroblast senescence in geriatric skin results in a greater reliance of epidermal keratinocytes on a more mutagenic form of DNA synthesis following UVB exposure that ultimately predisposes the cells to carcinogenesis. This translational project will be carried out in collaboration with dermatologist Dr. Jeffrey Travers. This project was previously funded by a VA Clinical Merit Award.
Project 4: Damaged cell-free DNA. Cell-free DNA (cfDNA) found in biofluids is increasingly being used in the diagnosis and treatment of a variety of disease states. Though DNA is known to be susceptible to damage by a large number of genotoxic agents, the presence of DNA lesions in cfDNA has thus far not been examined. We have been characterizing bulky adduct-containing cell-free DNA (termed "bad-cfDNA") released from cultured cells in vitro and human skin following exposure to environmental carcinogenes such as UVB radiation and benzo[a]pyrene and DNA damaging cancer chemotherapy drugs. We hypothesize that DNA adducts limit the degradation of EV-associated DNA and impact the function of the DNA once taken up by bystander cells. This project is currently funded by the US-Israel Binational Science Foundation.
Teaching
PTX-7002: Journal Club/Special Topics: Genetic Toxicology (1 cr hr, Fall)
PTX-7030: Genetic Toxicology Laboratory (3 cr hr, TBD)
PTX-7031: Circadian Medicine and Chronopharmacology (3 cr hr, TBD)
PTX-8007: Career Planning in Pharmacology & Toxicology (1 cr hr, TBD)
PTX-8020: Genetic Toxicology (3 cr hr, Spring)
PTX-8070: Cell Culture Training (1 cr hr, Fall)
Students Advised
My laboratory trains postdoctoral researchers as well as graduate students in the Pharmacology and Toxicology Master's Degree Program and the Biomedical Sciences Ph.D. Program.
Current Lab Members:
- Sri Meghana Yerrapragada, MS (Biomedical Sciences PhD; 2022-present)
- Pooja Chavda (Pharm/Tox MS; 2025-present)
- Udaya Byreddy (Pharm/Tox MS; 2025-present)
- Vijayalakshmi Jogi (Biomedical Sciences PhD; 2025-present)
- Charutha Raji (Pharm/Tox MS; 2025-present)
- Maneesha Panguluru (Pharm/Tox MS; 2025-present)
Previous Trainees and Lab Members:
- Alex Carpenter, PhD (Postdoctoral Researcher; Jan 2020-Dec 2025)
- Dean Rider, PhD (Research Assistant Professor; Feb 2023-Sept 2025)
- Dibya Guragai (Pharm/Tox MS; 2024-2025)
- Sameer Cholakkathody (Pharm/Tox MS; 2024-2025)
- Angitha Nair (Pharm/Tox MS; 2024-2025)
- Eniola Alabi (Pharm/Tox MS; 2024-2025)
- Irusha Dahal (Pharm/Tox MS; 2024-2025)
- Usha Atluri (Pharm/Tox MS; 2024-2025)
- Billy Cvammen, PhD (Biomedical Sciences PhD; 2020-2024)
- Aleena Alex, PharmD (Pharm/Tox MS; 2023-2024)
- Hrishikesh Kadam (Pharm/Tox MS; 2022-2023)
- Prashant Gaikwad (Pharm/Tox MS; 2022-2023)
- Swathi Kavuri, PharmD (Pharm/Tox MS; 2022-2023)
- Saman Khan, PhD (Postdoctoral; 2021-2022)
- Nadeen Anabtawi, PharmD (Pharm/Tox MS; 2019-2021)
- Vivek Gogusetti (Pharm/Tox MS; 2019-2021)
- Meghana Ginugu (Pharm/Tox MS; 2019-2021)
- Amber Castellanos (Anatomy MS; 2019-2020)
- Mariyyah Madkhali (Pharm/Tox MS: 2018-2020)
- Abdulrahman Alkawar (Pharm/Tox MS: 2018-2020)
- Rebekah Hutcherson (Biochem/Mol Bio BS Honors: 2018-2019)
- Kavya Shaj, PharmD (Pharm/Tox MS: 2017-2019)
Students wanting to carry out thesis research in my lab are strongly encouraged to take PTX7002 (Journal Club: Intro to Genetic Toxicology; Fall Semester) and PTX8020 (Genetic Toxicology; Spring semester), which provide background context and knowledge related to the types of research being performed in my laboratory. Thus, students taking these courses will be better acquainted with my research program and may be given priority for admittance as a graduate student in my lab. For more information about suggestions and expectations for students working in my lab, see the "Kemp Laboratory Handbook" file at the bottom of the screen.
Service
- WSU Faculty Senator (appointed; Spring 2026)
- WSU Graduate Faculty Membership Committee (Chair
- WSU Scholarship and Sponsored Research Committee
- Environmental Mutagenesis and Genomics Society
- Awards & Honors Committee (2019-2025; Chair)
- Councilor (2026-2029)
- Education Commitee, WSU Dept of Pharmacology & Toxicology
- Curriculum Commitee, WSU Biomedical Sciences PhD Program
News
- Sept 2025: Dibya successfully defends her MS thesis!
- Sept 2025: Drs. Kemp and Carpenter attend the annual EMGS meeting in Rochester, NY; Alex wins a best poster award!
- Aug 20, 2025: Dean's paper on TLS polymerase expression in young and geriatric skin is accepted for publication in JID Innovations!
- July 2025: Sameer defends his MS thesis
- June 19, 2025: Sri's paper on cell-free DNA release from cisplatin-treated cells is accepted for publication in DNA Repair!
- June 2025: Eniola successfully defends her MS thesis!
- May 2025: Dr. Kemp, Sri, and Eniola attend the Annual Midwest DNA Repair Symposium in Ann Arbor, Michigan.
- Apr 2025: Usha, Angitha, and Irusha successfully defend their MS theses!
- Jan 2025: Vijayalkshmi joins the lab as a BMS PhD student.
- Jan 2025: Udaya and Pooja join the lab to carry out their Pharm/Tox MS thesis research.
- Aug 2024: Alex's NIEHS K99 grant is awarded!
- June 2024: Billy's paper on circadian gene expression in human skin is accepted for publication in the Journal of Investigative Dermatology!
- June 2024: Alex, Sri, and Dr. Kemp attend the 25th Anniversary Midwest DNA Repair Symposium in Louisville, KY.
- May 2024: Dibya and Sameer join the lab to carry out their MS thesis research.
- Jan 2024: Angitha, Eniola, Usha, and Irusha join the lab to carry out their MS thesis research.
- Jan 3, 2024: Alex's methods paper on detecting extracellular DNA damage is accepted for publication in STAR Protocols!
- Nov 21, 2023: Billy's paper on circadian clock gene expression in young and geriatric skin is accepted for publication in the Journal of Investigative Dermatology!
- Sept 9-13, 2023: Dr. Kemp, Billy, and Alex attend the Environmental Mutagenesis and Genomics Society meeting in Chicago!
- May 5-7, 2023: Dr. Kemp, Billy and Sri attend the 22nd Annual Midwest DNA Repair Symposium in Iowa City!
- Apr 14/21, 2023: Hrishikesh, Swathi, and Prashant defend their masters theses!
- Feb 2023: Dr. Dean Rider joins the lab; Aleena joins the lab to carry out her MS thesis research.
- Dec 14, 2022: Our paper showing that cathepsin L artifactually cleaves nuclear proteins during the lysis of quiescent cells is accepted in microPublication Biology!
- Aug 27-Sept 1, 2022: Dr. Kemp, Alex, and Billy attend the 13th International Conference on Environmental Mutagens in Ottawa, Canada.
- Jun 9, 2022: Alex's paper on extracellular CPDs is published in the Journal of Investigative Dermatology!
- May 18-21, 2022: Drs. Kemp and Carpenter attend the annual meeting of the Society for Investigative Dermatology in Portland, Oregon
- Apr 18, 2022: Postdoc Dr. Alex Carpenter recieves a Mary Kay research grant!
- Mar 31, 2022: Our collaborative paper with Jun-Hyuk Choi is accetped for publication in Nucleic Acids Research!
- Mar 29, 2022: Our paper on the flavonoid nobiletin and its impact on UV responses is accepted for publication in Photochemistry & Photobiology!
- Jan 2022: Swathi, Hriskikesh, and Prashant join the lab to carry out their masters thesis research.
- Dec 2021: The Kemp laboratory moves to new, larger laboratory space!
- Dec 2, 2021: Our paper on XPA cleavage by cathepsin L during lysis of quiescent cells is accepted for publication in DNA Repair!
- Sept 9, 2021: Nadeen's paper on the effect of circadian clock modulating drugs on cisplatin responses is published in Scientific Reports!
- July 23, 2021: Alex wins a poster award at the ASBMB virtual meeting "Extracellular vesicle studies: from benchtop to therapeutics"!
- June 6, 2021: A review article on the circadian clock in the skin written by MD/MS student Janet Lubov, BMS PhD student Billy Cvammen, and Dr. Kemp has been accepted for publication!
- May 2021: All lab members are vaccinated against SARS-CoV-2!
- May 8, 2021: Alex gave an excellent plenary talk at the virtual SID meeting.
- April 30, 2021: Billy passes his preliminary exam!
- April 27, 2021: Nadeen defends her master's thesis!
- April 16, 2021: Vivek defends his master's thesis!
- April 15, 2021: Meghana defends her master's thesis!
- Mar 18, 2021: Our manuscript showing PCNA ubiquitination in human skin has been accepted for publication in the Journal of Biological Chemistry!
- Febr 11, 2021: Alex's abstract was selected for a plenary talk at the Society for Investigative Dermatology (Virtual) Meeting in May!
- Feb 1, 2021: Dr. Saman Khan joins the Kemp lab as a Postdoctoral Researcher!
- Aug 2021: Dr. Kemp gives a talk at the Environmental Mutagenesis and Genomics Society virtual meeting
- July 1, 2020: Dr. Kemp's grant proposal "Circadian Modulating Drugs in Cancer Prevention and Treatment was selected for funding by Ohio Cancer Research!
- May 13-16, 2020: Dr. Kemp gives a talk at the 2020 Society for Investigative Dermatology Meeting Virtual Conference.
- Oct 2020: The Kemp laboratory officially opens!
Publications
(2017-present)
- Khan S, Nair A, Carpenter MA, and Kemp MG. (2026). RAD51 and RAD51 paralog inhibition sensitizes nonreplicating quiescent keratinocytes to UV radiation. Photochemistry % Photobiology. In press
- Park Y, Sahu RP, Rohan CA, Kemp MG, Thyagarajan A, and Travers JB. (2025). Evidence for keratinocyte-derived microvesicle particles as carriers for the potent lipid mediator platelet-activating factor as effectors for systemic effects associated with many environmental stressors. J Investigative Dermatology. In press
- Annamraju R, Owens MS, Thyagarajan A, Corbin DA, Sherwin CMT, Bryant J, Fisher GW, Owens WR, Ketter A, Umerani A, Rohan CA, Kemp MG, Crow RK, Travers JB. (2025-in press). Possible involvement of keratinocyte-derived microvesicle particles in human photosensitivity disorders. Photochemistry & Photobiology. In press.
- Rider SD Jr, Owens MS, Rohan CA, Travers JB, and Kemp MG. (2025). Translesion synthesis DNA polymerase gene expression is impacted by age and IGF-1 in epidermal human skin. JID Innovations. 5(6): 100409.
- Yerrapragada SM, Alex A, Adar S, Kemp MG, and Carpetner MA. (2025). Treatment of human cells with the anti-cancer drug cisplatin results in the caspase-dependent release of adduct-containing cell-free DNA. DNA Repair. 151:103855.
- Cvammen W and Kemp MG. (2024). Analysis of circadian clock gene expression in human skin explants. JID Innovations. 4(6): 100308.
- Cvammen W, Rider SD Jr, Kemp MG. (2024). Analysis of circadian clock gene expression in epidermal human skin indicates that body location impacts rhythm amplitude. Journal of Investigative Dermatology. 144(11): 2599-2602.
- Chiou YY and Kemp MG. (2024). RNA polymerase tracking along damaged DNA: Impact on DNA repair and mutagenesis. Proceedings of the National Academy of Sciences USA. 121(23): e2408073121.
- Carpenter MA, Thyagarajan, A, Owens M, Annamraju R, Borchers CB, Travers JB, and Kemp MG. (2024). The acid sphingomyelinase inhibitor imipramine enhances the release of UV photoproduct-containing DNA in small extracellular vesicles in UVB-irradiated human skin. Photochemistry & Photobiology. 100(6): 1894-1901.
- Cvammen W and Kemp MG. (2024). The REV-ERB antagonist SR8278 modulates keratinocyte viability in response to UVA and UVB radiation. Photochemistry & Photobiology. 100(6): 1864-1873.
- Christian L, Manjrekar P, Henkels KM, Rapp CM, Annamraju R, Lohade R P, Singh S, Carpenter M A, Khan S., Kemp MG, Chen Y, Sahu RP, and Travers JB. (2024). Evidence for the involvement of keratinocyte-derived microvesicle particles in the photosensitivity associated with xeroderma pigmentosum type A deficiency. Photochemistry & Photobiology. 100(5): 1457-1466.
- Carpenter MA, Yerrapragada S, Alex, A, Kemp MG. (2024). Protocol for immunoblot detection of UVB photoproducts in extracellular DNA. STAR Protocols. 5(1): 102838.
- Cvammen W, Rider SD, Travers SB, and Kemp MG. (2024). Effects of Age and Sex on the Expression of Core Circadian Clock Genes in Human Skin Epidermis. Journal of Investigative Dermatology. 144(5): 1372-1378.
- Gaikwad P and Kemp MG. (2022). Cathepsin L inhibition prevents the cleavage of mulitple nuclear proteins upon lysis of quiescent human cells. MicroPublication Biology.
- Carpenter MA, Ginugu M, Khan S, and Kemp MG. (2022). DNA containing cyclobutane pyrimidine dimers is released from UVB-irradiated keratinocytes in a caspse-dependent manner. J Investigative Dermatology. 142(11): 3062-3070.
- Yan S, Zho J, Kemp M, Sobol RW. (2022). Editorial: Mechanistic studies of genome integrity, environmental health, and cancer etiology. Front Cell Dev Biol. 10:1026326.
- Kim SH, Kim GH, Kemp MG, and Choi JH. (2022). TREX1 degrades the 3' end of the small DNA oligonucleotide products of nucleotide excision repair in human cells. Nucleic Acids Research. 50(7): 3974-3984.
- Cvammen W and Kemp MG. (2022). Flavonoid nobiletin exhibits differential effects on cell viability in keratinocytes exposed to UVA versus UVB radiation. Photochemistry & Photobiology. 98(6): 1372-1378.
- Frommeyer TC, Rohan CA, Spandau DF, Kemp MG, Wanner MA, Tanzi E, and Travers JB. (2022). Wounding therapies for prevention of photocarcinogenesis. Frontiers in Oncology. 11: 813132.
- Khan S, Cvammen W, Anabtawi N, Choi JH, and Kemp MG. (2022). XPA is susceptible to proteolytic cleavage by cathepsin L during lysis of quiescent cells. DNA Repair. 109: 103260.
- Mahajan AS, Arikatla VS, Thyagarajan A, Zhelay T, Sahu RP, Kemp MG, Spandau DF, and Travers JB. (2021). Creatine and nicotinamide prevent oxidant-induced senescence in human fibroblasts. Nutrients. 13(11): 4102.
- Spandau DF, Chen R, Wargo JJ, Rohan CA, Southern D, Zhang A, Loesch M, Weyerbacher J, Tholpady SS, Lewis DA, Kuhar M, Tsai KY, Casellanos AJ, Kemp MG, Markey M, Cates E, Williams AR, Knisely C, Bashir S, Gabbard R, Hoopes R, and Travers JB. (2021). Randomized control trial of fractionated laser resurfacing on aged skin as prophylaxis against actinic neoplasia. J Clinical Investigation. 131(19): e150972.
- Anabtawi N, Cvammen W, and Kemp MG. (2021). Pharmacological inhibition of cryptochrome and REV-ERB promotes DNA repair and cell cycle arrest in cisplatin-treated human cells. Scientific Reports. 11(1): 17997.
- Carpenter MA and Kemp MG. (2021). Topical treatment of human skin and cultured keratinocytes with high-dose spironolactone reduces XPB expression and induces toxicity. JID Innovations. 1(3): 100023.
- Sarkar S, Porter KI, Dakup PP, Gajula RP, Koritala BSC, Hylton R, Kemp MG, Wakamatsu K, and Gaddameedhi S. (2021). Circadian clock protein BMAL1 regulates melanogenesis through MITF in melanoma cells. Pigment Cell Melanoma Res. 34(5): 955-965.
- Spandau DF, Chen R, Wargo JJ, Rohan CA, Southern D, Zhang A, Loesch M, Weyerbacher J, Thopady SS, Lewis DA, Kuhar M, Tsai KY, Castellanos AJ, Kemp MG, Markey M, Cates E, Williams AR, Knisely C, Bashir S, Gabbard R, Hoopes R, and Travers JB. (2021). Randomized controlled trial of fractionated laser resurfacing to aged skin as prophylaxis against actinic neoplasia. J Clin Invest. 24: 149072.
- Lubov JE, Cvammen W, and Kemp MG (2021). The Impact of the Circadian Clock on Skin Physiology and Cancer Development. Int J Mol Sci. 22(11): 6112.
- Liu L, Awayemi AA, Fahy KE, Thapa P, Borchers C, Wu BY, McGlone CL, Schmeusser B, Sattouf Z, Rohan CA, Williams AR, Cates EE, Knisely C, Kelly LE, Bihl J, Cool DR, Sahu RP, Wang J, Chen Y, Rapp CM, Kemp MG, Johnson RM, and Travers JB. (2021). Keratinocyte-derived microvesicle particles mediate ultraviolet B radiation induced system immunosuppression. J Clinical Investigation. 131(10): e144963.
- Hutcherson RJ, Gabbard RD, Castellanos AJ, Travers JB, and Kemp MG. (2021). Age and insulin-like growth factor-1 impact PCNA mono-ubiquitination in UVB-irradiated human skin. J Biol Chem. 100570. YouTube summary: click here.
- Ume AC, Pugh JM, Kemp MG, Williams CR. (2020). Calcineurin inhibitor (CNI)-associated skin cancers: New insights on exploring mechanisms by which CNIs downregulate DNA repair machinery. Photoderm Photoimmunol Photomed. 36(6): 433-440.
- Alkawar AMM, Castellanos AJ, Carpenter MA, Hutcherson RJ, Madkhali MAO, Johnson RM, Bottomley M, and Kemp MG. (2020). Insulin-like Growth Factor-1 Impacts p53 Target Gene Induction in UVB-irradiated Keratinocytes and Human Skin. Photochemistry & Photobiology. 96(6): 1332-1341. YouTube summary: click here.
- Gabbard RD, Hoopes RR, and Kemp MG. (2020). Spironolactone and XPB: An Old Drug with a New Molecular Target. Biomolecules. 10(5): 756.
- Choi J-H, Han S, and Kemp MG. (2020). Detection of the small oligonucleotide products of nucleotide excision repair in UVB-irradiated human skin. DNA Repair. 86: 102766. YouTube summary: click here.
- Travers JB, Kemp MG, Weir NM, Cates E, Alkawar AM, Majahan AS, Spandau DF. (2020). Wounding with a microneedling device corrects the inappropriate ultraviolet B radiation response in geriatric skin. Arch Dermatol Res. 312(1): 1-4.
- Shaj K, Hutcherson RJ, Kemp MG. (2020). ATR kinase activity limits mutagenesis and promotes the clonogenic survival of quiescent human keratinocytes exposed to UVB radiation. Photochemistry & Photobiology. 96(1): 105-112. YouTube summary: click here.
- Hutcherson RJ and Kemp MG. (2019). ATR kinase inhibition sensitizes quiescent human cells to the lethal effects of cisplatin but increases mutagenesis. Mutation Research. 816-818: 111678. YouTube summary: click here.
- Kemp MG. (2019). Damage removal and gap filling in nucleotide excision repair. The Enzymes: DNA Repair, 45: 59-97.
- Kemp MG, Krishnamurthy S, Kent MN, Schumacher DL, Sharma P, Excoffon KJDA, Travers JB. (2019). Spironolactone depletes the XPB protein and inhibits DNA damage responses in UVB-irradiated human skin. J Invest Dermatology.139(2): 448-454. YouTube summary: click here.
- Khan AQ, Travers JB, and Kemp MG. (2018). Roles of UVA Radiation and DNA Damage Responses in Melanoma Pathogenesis. Environmental and Molecular Mutagenesis, 59(5): 438-60.
- Poudel S, Yao J, Kemp MG, Leffak M. (2018). Interaction between DUE-B and Treslin is required to load Cdc45 on chromatin in human cells. J Biol Chem. 293(37): 14497-14506.
- Dakup PP, Porter KI, Little AA, Gajula RP, Zhang H, Skorynakov E, Kemp MG, Van Dongen HPA, and Gaddameedhi S. (2018). The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget. 9(18): 14524-14538.
- Baek S, Han S, Kang D, Kemp MG*, Choi JH. (2018). Simultaneous detection of nucleotide excision repair events and apoptosis-induced DNA fragmentation in genotoxin-treated cells. Scientific Reports. 8(2265): 1-11. (*co-corresponding author) YouTube summary: click here.
- Kemp MG, Spandau DF, and Travers JB. (2017). Impact of Age and Insulin-like Growth Factor-1 on DNA Damage Responses in UV-irradiated Human Skin. Molecules. 22(3).
- Kemp MG. (2017). DNA damage-induced ATR kinase activation in non-replicating cells is regulated by the XPB subunit of transcription factor II-H (TFIIH). J Biol Chem. 292(30): 12424-12435.
- Kemp MG, Spandau DF, Simman R, and Travers JB. (2017). Insulin-like Growth Factor-1 Receptor Signaling is Required for Optimal ATR-CHK1 Kinase Signaling in Ultraviolet B (UVB)-irradiated Human Keratinocytes. J Biol Chem. 292(4): 1231-9. YouTube summary: click here.
- Kemp MG and Hu J. (2017). Post-Excision Events in Human Nucleotide Excision Repair. Photochemistry & Photobiology. 93(1): 178-91.
- Song J, Kemp MG and Choi J-H. (2017). Detection of the Excised, Damage-containing Oligonucleotide Products of Nucleotide Excision Repair in Human Cells. Photochemistry & Photobiology. 93(1): 192-8.
Check out my full list of publications on PubMed.
Awards/Recognition
- Oct 2024-Sept 2028: US-Israel Binational Science Foundation Award 2023277 - "Analysis and function of damaged cell-free DNA"
- Apr 2023: Junior Faculty Award, Wright State University Academy of Medicine
- Feb 2023-Jan 2024: DoD Defense University Research Instrumentation Program (DURIP) Award FA9550-23-1-0080
- Oct 2021-Sept 2025: VA Clinical Merit Award CX002241 - "Mapping DNA Repair and Error-Prone DNA Synthesis in Geriatric Skin"
- July 2020-June 2022: Ohio Cancer Research Grant #5020 - "Circadian Clock Modulating Drugs in Cancer Prevention and Treatment"
- May 2019: Newly Independent Investigators Engagement Program, Environmental Mutagenesis and Genomics Society
- Feb 2019-Jan 2025: NIGMS R01 Award GM130583 - "DNA Damage Kinase Signaling in Non-replicating Human Cells and Tissues"
- Mar 2018: Travel Award, Society for Investigative Dermatology
- Sept 2016: Emerging Scientist Travel Award, Environmental Mutagenesis and Genomic Society
Professional Affiliations/Memberships
- American Society for Biochemistry and Molecular Biology (ASBMB), 2015-present
- Environmental Mutagenesis and Genomics Society (EMGS), 2016-present
- American Society for Photobiology (ASP), 2016-present
- Society for Investigative Dermatology (SID), 2017-present